ICN2 group leader ICREA Prof. Jose Antonio Garrido and fellow Graphene Flagship programme coordinator, Prof. Kostas Kostarelos of the University of Manchester, were invited by Advanced Materials to present their vision of the role of graphene in the design of advanced brain-computer interfaces. Published earlier this month, the work entitled “Graphene in the design and engineering of next-generation neural interfaces” outlines the current state-of-the-art in this field, along with the ways graphene is allowing us to overcome some of the obstacles to fully realising the next generation of neural interfaces.
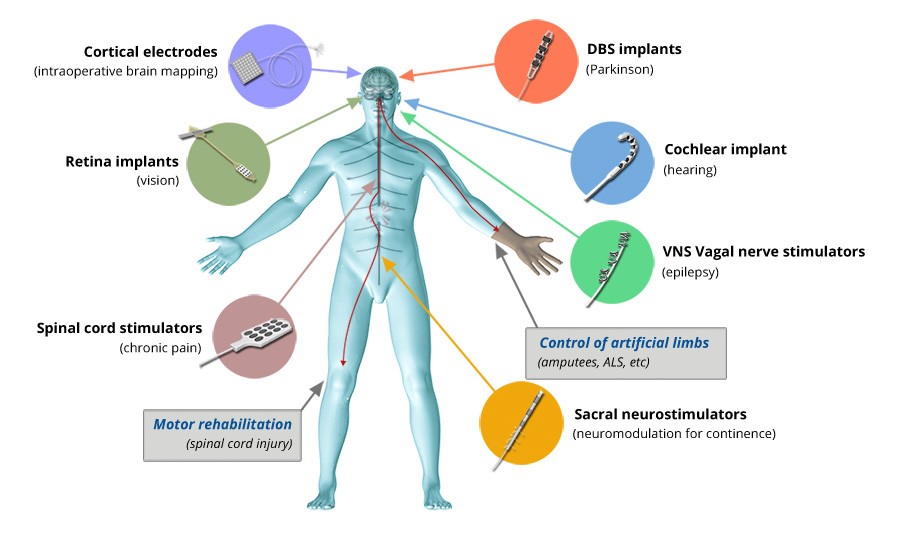
Deep brain stimulation to treat the symptoms of motor disorders like Parkinson’s disease, retinal and cochlear implants to help restore sight and sound, and nerve interfaces that allow users sensory feedback and enhanced mechanical control over their artificial limbs… These are some more well-known applications of electrically-functional implantable neural interfaces, but there are others. Such devices are fast becoming an indispensable tool for all manner of clinical interventions that require stimulation, recording and/or understanding of the electrical activity of the nervous system.
Having recently embarked on a biomedical research programme within the European Commission’s Graphene Flagship project, Prof. Kostas Kostarelos of the University of Manchester and ICREA Prof. Jose Antonio Garrido, leader of the ICN2 Advanced Electronic Materials and Devices Group are well-qualified to bring us up to speed with the current state-of-the-art in neural interfaces. Looking through the lens of graphene as a key enabling material taking these technologies to the next level, they present the different challenges and applications currently being addressed in this field. Together with co-authors Dr. Clement Hebert (ICN2) and Dr. Melissa Vincent (Uni. Manchester), the review was invited for publication in Advanced Materials.
The challenges
Given the hugely intricate nature of the human nervous system, neural implants must be as minimally-invasive as possible, allow for an easy surgical procedure, and provide efficient and consistent activity for the duration of their functional lifetime. Recording capabilities need to be such that they allow the detection of signals from individual neurons and neural ensembles over large areas and with a high spatial resolution. For stimulation applications, a suitable charge injection capacity is required in order to elicit a response from the tissue being targeted without causing damage. Additionally, devices must be biocompatible and mechanically compliant with neural tissues, and be stable enough for use in long-term implants.
Enter graphene...
Graphene is known to be biocompatible with neural cells and its capacity to effectively interface with neuronal tissue allows for both recording and electrical stimulation functionalities at the desired levels. In fact, where current neural interfaces are designed to perform a single function, either recording or electrically stimulating brain tissue, graphene-based alternatives can combine both functions in a single device, even adding a few more into the mix.
Graphene-based materials for neural stimulation: Recent studies have demonstrated the efficacy of 3D or structured porous graphene films in the stimulation of neural tissue, presenting superior charge injection capacities than competing materials.
Graphene for electrical neural recording: Again, the use of structured or porous films has been shown to improve the performance of graphene-based materials for recording applications. Here, increasing the specific surface area of the electrode serves to reduce impedance and, by doing so, optimises the signal-to-noise ratio. Unlike standard metals used in neural interfaces, graphene can also be used to fabricate sensors based on the advantageous field-effect transistor (FET) configuration, which further reduces sensitivity to external noise.
Graphene for controlled drug delivery at the neural interface: Graphene and its derivatives are being actively studied for their potential as nanocarriers in the targeted delivery and intracellular transport of therapeutic agents. Strategies are being devised to engineer smart coatings for neural implants that allow the in situ, stimulation-triggered release of dopamine, microglial inhibitors and anti-inflammatory agents. Such coatings could also be used to deliver biologically-active molecules that improve surface softness and overall biocompatibility of the implant.
Graphene for in situ biosensing: The electronic properties and high porosity of graphene are such that it has been extensively studied in the context of biosensing applications, and a broad variety of devices exhibiting high sensitivity, low noise and low detection thresholds have already been reported. These will allow simultaneous mapping of the electrical activity, biochemistry and metabolic activity of neural tissue, conferring a greater understanding of neurological disorders and tissue response to treatment.
To conclude, the remarkable combination of physical, chemical, structural and electronic properties presented by graphene-based materials places this 2D carbon allotrope at the heart of neural interface design going forwards. The next generation of neural interfaces will combine multiple functionalities in a way that is expected to completely transform the diagnosis, treatment and monitoring of neurological and related disorders.
Article reference:
K. Kostarelos, M. Vincent, C. Hebert, J. A. Garrido. Graphene in the design and engineering of next-generation neural interfaces. Advanced Materials. 2017, 1700909. https://doi.org/10.1002/adma.201700909

