Monday, 21 September 2020
Nanophotonic biosensors for rapid diagnostics of COVID-19 and other respiratory virus infections
In a thorough review recently published in ACS Sensors, researchers from the ICN2 NanoBiosensors and Bioanalytical Applications group, led by Prof. Laura M. Lechuga, provide an overview of the current diagnostic techniques for respiratory viruses, as the SARS-CoV-2, causing agent of COVID-19, and discuss how nanophotonic biosensor technologies can contribute to the development of new fast, highly sensitive and affordable diagnostic tests.
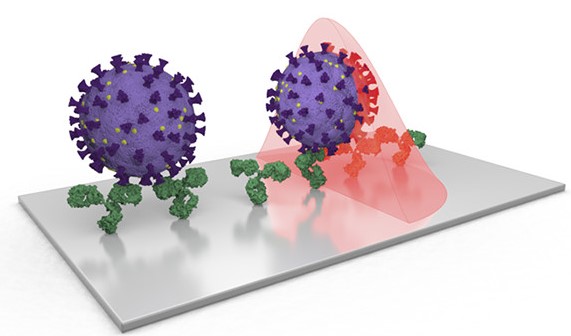
The swift worldwide spread of the COVID-19 disease, caused by the respiratory virus SARS-CoV-2, has revealed the difficulties of facing such a pandemic and has driven a race to find new specific solutions in terms of diagnostics, therapy and prevention. While effective treatments and vaccines are certainly essential, fast and reliable diagnosis methods are key to monitoring and containing the diffusion of the infection.
An accurate and explanatory review recently published in ACS Sensors and signed by members of the ICN2 NanoBiosensors and Bioanalytical Applications group, led by Prof. Laura M. Lechuga, discusses current diagnostic techniques for COVID-19 and possible future developments in this field. In particular, after highlighting the advantages and limitations of the methods available at present, the article introduces optical biosensors and illustrates how nanophotonic label-free devices can contribute to meet the emerging needs in this context.
Diagnostic techniques for respiratory diseases as COVID-19 are based on three possible approaches, which detect either the genome (i.e. the nucleic acid) of the virus, or its antigens (thus, the entire virus or fragments of it), or the antibodies produced by the infected host (commonly called serological analysis). The most reliable method for COVID-19 diagnosis is still the polymerase chain reaction (PCR) test — i.e. molecular swabs — which uses the first approach and provides the required sensitivity and specificity. Though, the analysis revealing the infection has to be carried out in specialised laboratories and it is relatively slow (the results are normally available between 12 and 48 hours after the organic sample has been taken). More rapid options employ the lateral flow technology instead: even though ideal for point-of-care diagnostics, they suffer from insufficient sensitivity and can provide only qualitative (yes/no), not quantitative information about a virus infection.
It is therefore evident that a rapid, accurate and affordable method for point-of-care diagnostics is strongly needed to be able to monitor and contain the spread of COVID-19, as well as of possible future diseases caused by other coronaviruses. It is also desirable to have a single platform integrating all three aforementioned diagnostic strategies, combining the best features of each of them.
As explained in this review, optical biosensors are excellent candidates for this application, thanks to their high sensitivity, robustness, miniaturisation and integration capability, as well as portability. They measure variations of the optical properties of propagated light when the recognition elements (antibodies, nucleic acid sequences, enzymes, proteins, etc.) interact with the target analyse in the sample. Among them, photonics and plasmonic biosensors based on the evanescent field sensing principle have already been largely applied, both in pharmaceutical and clinical scenarios, and have demonstrated that this technology is mature and reliable. These biosensors can operate in a label-free configuration and, thanks to their remarkable sensitivity, they are effective on very small samples (without the need for replicating the organic material to analyse). In addition, several targets can be detected simultaneously by implementing different sensing channels on the same device.
Nanophotonic biosensors present indeed the potential to combine rapid virus detection and identification, genomic analysis, and even serological assay in one integrated platform. This is the goal set by the CoNVat project (Combating 2019-nCoV: Advanced nanobiosensing platforms for POC global diagnostics and surveillance), funded by the European Commission under the Express Coronavirus Call launched in February 2020 and led by Prof. Laura M. Lechuga, which aims to develop a point-of-care device employing a silicon interferometric sensor for SARS-CoV-2 infection diagnostics. While the primary objective of this project is to provide a fast, reliable, portable and cheap test system to detect the intact virus in nasopharyngeal and saliva samples, Prof. Lechuga’s team is also working on applying this technique to the detection of the virus genome and antibodies.
Reference article:
Maria Soler, Maria Carmen Estevez, Maria Cardenosa-Rubio, Alejandro Astua, and Laura M. Lechuga, How Nanophotonic Label-Free Biosensors Can Contribute to Rapid and Massive Diagnostics of Respiratory Virus Infections: COVID-19 Case; ACS Sensors, August 2020. DOI: 10.1021/acssensors.0c01180

