ICN2 scientists have participated in an international study to develop a new water electrolysis system based on a cobalt tungstate membrane. This type of catalyst could be the key to the cheap and sustainable production of hydrogen, a potential green fuel of the future. The research also involved infrastructure from the InCAEM project at the ALBA Synchrotron.
The journal Science recently published the results of an international study in which ICN2 Advanced Electron Nanoscopy Group members, led by ICREA Prof. Jordi Arbiol, participated. This research has demonstrated the usefulness of a novel cobalt tungstate electrocatalyst to carry out the electrolysis reaction of water under industrial conditions. These outcomes pave the way to producing hydrogen more cleanly and sustainably.
The structural and chemical characterization of this material has been carried out by using the electron microscope EM02-METCAM, co-funded with European Regional Development Funds (ERDF) and located at the Joint Electron Microscopy Center at ALBA Synchrotron (JEMCA). The analyses were carried out by David Llorens Rauret, Dr Bernat Mundet and Dr Alba Garzón Manjón, and included state-of-the-art techniques to visualize oxygen atoms and detect the cobalt tungstate vacancies that appeared after catalyst activation. The overall work has been led by Prof. Pelayo García de Arquer’s research group at the Institute of Photonic Sciences (ICFO).
These results are just the starting point of the upcoming opportunities of the InCAEM project. This new in situ correlative facility for advanced energy materials will provide unique equipment on the frontier of knowledge.
The challenge of "green" hydrogen
Today, the climate crisis has highlighted the need to develop and improve more sustainable alternatives to fossil fuels. One of these potential energy sources is hydrogen (H2), which can be produced by water electrolysis. In this reaction, water molecules (H2O) are split into hydrogen and oxygen (O2) by passing an electric current.
Currently, the most common method of producing hydrogen is using natural gas, which results in the emission of polluting gases such as carbon dioxide and carbon monoxide. However, unlike this method, water electrolysis can produce hydrogen without harmful emissions or hazardous waste (green hydrogen). For this reason, this type of technology could play a key role in the fight against the climate crisis.
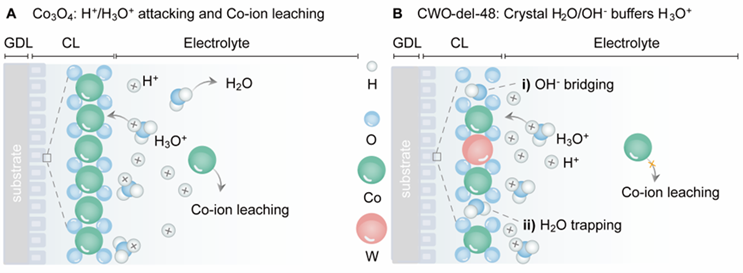
The platinum-free membranes' potential
This team of scientists has developed a novel electrolysis system based on a strategy called PEMWE (Proton Exchange Membrane Water Electrolysis). In this specific case, the catalyst is a cobalt tungstate membrane that acts trapping the water molecules and enhancing the proton exchange. Unlike other common water electrolysis systems, it does not use platinum, a very scarce material, which is important in reducing costs.
The catalytic reaction obtained with this system proved to be efficient and feasible under industrial conditions. The system achieved a current density of 1.8 A·cm-² a 2 V, and stable operation up to 1 A·cm² at industrial conditions. (80°C). These figures represent a milestone in the field of platinum-free PEMWEs.
These results reveal new information about the design of electrocatalysts to produce clean hydrogen at a large scale. Furthermore, it is noted that efforts are still needed in the research and development of new catalysts based on alternative materials, such as manganese or nickel, considering aspects such as geopolitical barriers and environmental factors related to the extraction and treatment of these elements.
Reference article
Ram, R., Xia, L., Benzidi, H., Guha, A., Golovanova, V., Garzón, A., Manjón, D. L., Rauret, P. S., Dimitropoulos, M., Mundet, B., Pastor, E., Celorrio, V., Mesa, C. A., Das, A. M., Pinilla-Sánchez, A., Giménez, S., Arbiol, J., López, N., & García de Arquer, F. P. (2024). Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis. Science. https://doi.org/10.1126/science.adk9849

