Researchers at ICN2 have developed a novel sensor that can detect hydrogen peroxide (H2O2) released by live cells in real time, offering a new tool for cancer cell monitoring. This innovative device uses a nanostructured film with nanozyme properties and is integrated into a nitrocellulose substrate that can host live cells.
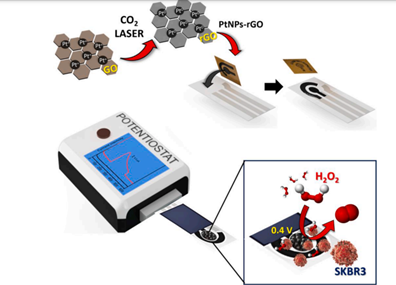
The journal Biosensors and Biolectronics has recently published a study carried out by researchers from the Nanobioelectronics and Biosensors Group, with the supervision of Dr Ruslan Alvarez Diduk and ICREA Prof. Arben Merkoçi, leader of the group. They have developed a new strategy for detecting tumour cells, based on a device that contains a nanometric film produced from reduced graphene oxide, decorated with platinum nanocubes. This sensor can detect hydrogen peroxide (H2O2) in real-time, a molecule produced by these cells when stimulated by stressors. This represents a novel strategy for developing point-of-care devices to detect or monitor the progression of diseases such as cancer or neurodegenerative disorders.
The research was a collaborative effort between Prof. Dario Compagnone, from the University of Teramo, and Prof. Carme Nogués, from the Universitat Autònoma de Barcelona (UAB).
Tumour cells and hydrogen peroxide production
H2O2 is a molecule that acts as a signalling molecule in certain cellular processes and is one of the substances produced by our metabolism. However, it is produced in very small amounts in healthy people. Altered production may indicate damage or altered processes at the cellular level. This is characteristic of certain disorders, including some degenerative diseases.
In the same way, cancer cells, which have a higher oxidative activity, may produce abnormal levels of H2O2 and other oxidative molecules. The altered release of this substance is therefore an indicator of the pathological state of the cells and can be used to determine whether they are cancerous.
How does this sensor work?
This study investigated the effectiveness of this novel system in analysing and detecting potentially cancerous cells. The sensor comprises a highly exfoliated, three-dimensional film produced from reduced graphene oxide, decorated with platinum nanocubes (cube-shaped structures of nanometric size). This film was in turn embedded in a nitrocellulose substrate capable of hosting the living cells to be analysed.
This system can act as a nanozyme. In other words, it is a nanomaterial with properties like a natural enzyme. In particular, the platinum particles can act similarly to peroxidase enzymes, which use peroxides such as H2O2 to carry out an oxidation-reduction reaction. Therefore, by analysing the electrochemical potential generated, it is possible to determine the concentration of H2O2.
To demonstrate its effectiveness, the researchers tested the sensor with two cell lines: Vero cells (non-tumorigenic) and SKBR3 cells (tumorigenic). The results successfully demonstrated the system's ability to detect, in real-time, the H2O2 molecules produced by tumour cells after stimulation with stressors. In this way, the H2O2 levels were able to identify whether the cells were tumour cells or not.
This work opens the door to new strategies for the cheap production of point-of-care diagnostic devices based on similar films, using only simple laboratory equipment. This would make it possible to monitor the status of potentially cancerous cells easily and economically.
Reference article:
Bukhari, UA; Della Pelle, F; Alvarez Diduk, R; Scroccarello, A; Nogués, C; Careta, O; Compagnone, D; Merkoci, A. Laser-assembled conductive 3D nanozyme film-based nitrocellulose sensor for real-time detection of H2O2 released from cancer cells. (2024). Biosensors and Bioelectronics. https://doi.org/10.1016/j.bios.2024.116544

