A new study by ICN2 researchers highlights how experimental factors such as temperature, measurement techniques, and data normalisation can significantly influence methane oxidation results. The findings underscore the need for standardised protocols to advance sustainable fuel research.
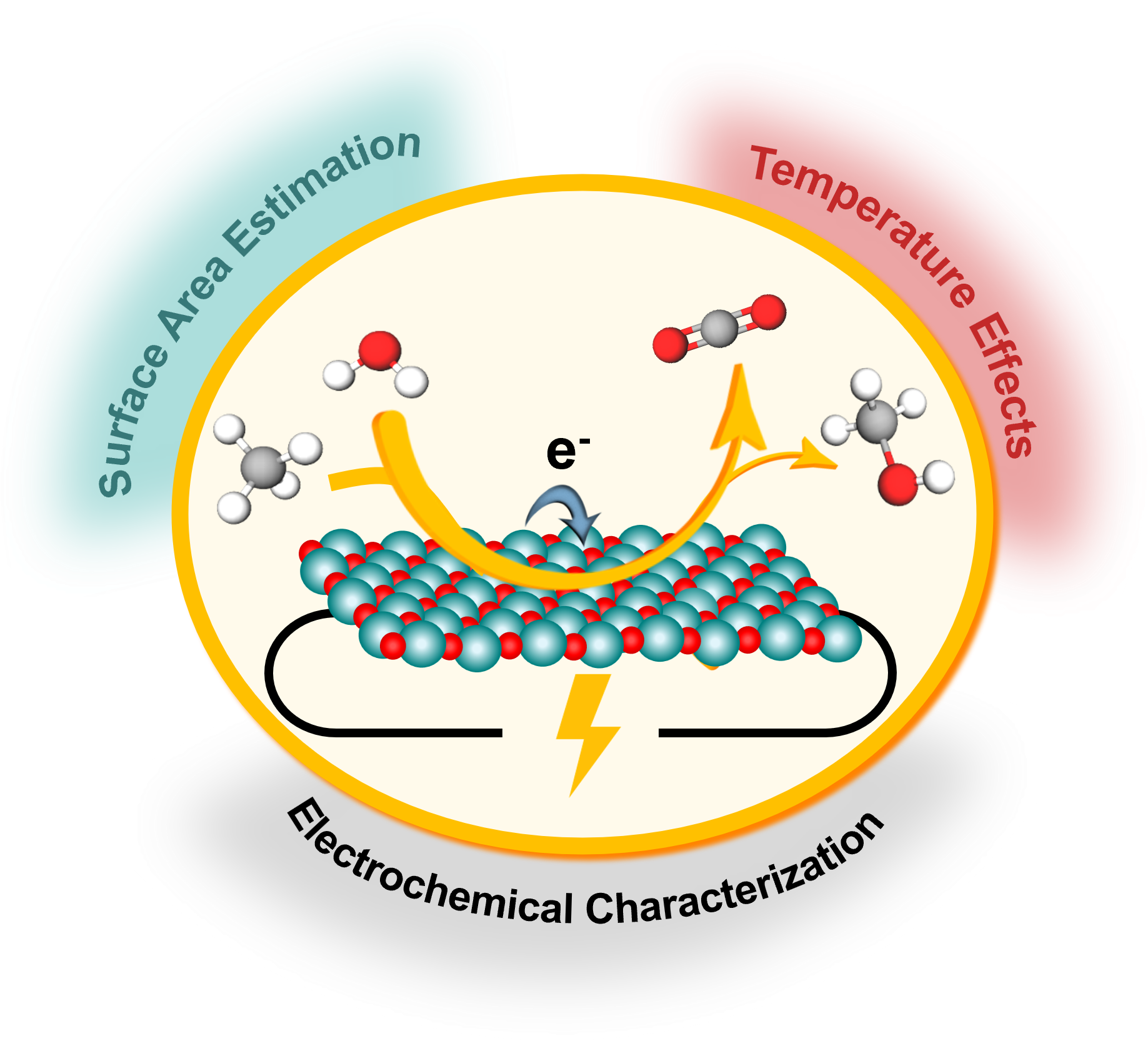
Methane, the main component of natural gas, is a potent greenhouse gas whose emissions constitute the second-largest contributor to global warming. Converting methane into methanol using electrochemical processes offers a dual strategy of reducing emissions and producing green fuels with renewable electricity. However, making this process efficient and profitable remains a major scientific and technological challenge.
In a recent study published in ACS Energy Letters, Dr Silvia Favero and ICREA Prof. María Escudero-Escribano (ICN2 NanoElectrocatalysis and Sustainable Chemistry Group), in collaboration with José Alejandro Arminio-Ravelo (formerly a PhD and postdoctoral researcher at the NanoESC Lab at the University of Copenhagen), examined how different experimental procedures can significantly influence results. One of the main obstacles at present is the lack of standardised protocols for carrying out these reactions.
To illustrate this issue, the team used iridium oxide as a model system — a well-known material that is widely used as an electrocatalyst in electrochemical reactions, including water oxidation for green hydrogen production. The experiments revealed that factors such as temperature, the electrochemical technique employed, or the method used to calculate the catalyst's active surface area can alter data interpretation considerably. Furthermore, many of these effects cannot be explained by the current understanding of electrochemical methane oxidation mechanisms and remain unclear, making it challenging to compare results across different laboratories.
To address these challenges, the authors propose three main strategies:
- Standardised measurement protocols. Start from a consistent initial state of the catalyst and normalise the current by its electrochemically active surface area. Report at least three independent measurements and their standard deviation and specify the exact testing protocol.
- Detailed studies of the reaction mechanism. Use in situ spectroscopic techniques to identify reaction intermediates and elucidate the reaction mechanism.
- Explore more experimental parameters. Beyond the parameters analysed in this study, investigate the influence of other factors, such as electrolyte composition or concentration, on the reaction.
Although this work does not yet solve the challenge of efficiently converting methane into methanol, it represents an important step forward. The researchers demonstrated that, without standardised protocols and more detailed studies, it will be impossible to reliably compare results and improve the design of highly effective catalysts.
Reference article:
Arminio-Ravelo, JA; Favero, S; Escudero-Escribano, M. Why Testing Protocols Matter in Electrochemical Methane Oxidation: Insights from IrOx in Acid. ACS Energy Letters. (2025). https://pubs.acs.org/doi/10.1021/acsenergylett.5c01848

