X-Ray diffraction facility - Case Studies
Malvern PANalytical X’pert Pro MPD
Case study 1
Authors: Vincent Guillerm, Heng Xu, Jorge Albalad, Inhar Imaz, and Daniel Maspoch
Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain
The objective of the present work was the postsynthetic generation of mesoporosity in metal–organic frameworks (MOFs) using ozone-based method developed in the Supramolecular nanochemistry and materials group (NANOUP). We carefully selected mixed-ligand Zr-fcu-MOFs based on organic ligand pairs in which one ligand has ozone-cleavable olefin bonds and the other ligand is ozone-resistant. Then, we were able to selectively break the cleavable ligand via ozonolysis to trigger fusion of micropores into mesopores within the MOF framework. Depending on the cleavable ligand used, the resultant ligand-fragments can be removed from the ozonated MOF by either washing or sublimation. Compared to the corresponding highly microporous starting MOFs, the highly mesoporous product MOFs exhibit radically distinct gas sorption properties although the crystallinity is totally preserved as we demonstrated using powder diffraction techniques.
Reference: Vincent Guillerm et al. J. Am. Chem. Soc., 140 (44), pp 15022–15030. 2018
DOI: 10.1021/jacs.8b09682

Malvern PANalytical X’pert Pro MRD
Case Study 2: Water uptake of cellulose thin films
Authors: Martin Kreuzer(1) and Monica Lira(2)
(1) ALBA Synchrotron Light Source, 08290 Cerdanyola del Vallès, Barcelona, Spain
(2) Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
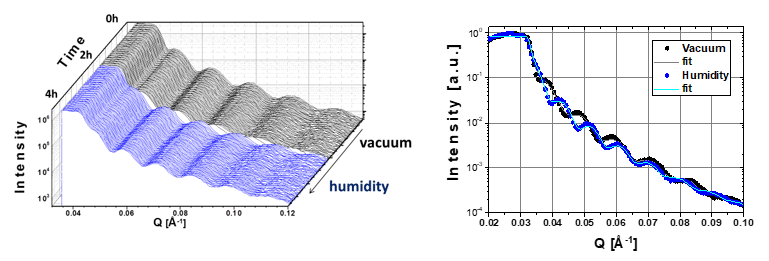
ICN2 Phononic and Photonic Nanostructures (P2N) Group have proved the water uptake of cellulose thin films using in-situ X-ray reflectometry.
Nanofibrillated cellulose, a polymer that can be obtained from one of the most abundant biopolymers in nature, is being increasingly explored due to its outstanding properties for packaging and device applications. Still, open challenges in engineering its intrinsic properties remain to address. Results show cellulose film thickness variations during water uptake at different humidity measured in-situ by XRR.
Measurements have been in vacuum and in a wet nitrogen flow using a graphene dome for atmospheric control.

First, a cellulose thin film has been monitored in vacuum for 2 hours, measuring a full reflectivity curve every 40 seconds. After 2 hours a wet nitrogen flow has been applied and the changes in reflectivity have been followed. The fitting of the reflectivity data of the thin film after 2 hours in vacuum and after 2 hours under a wet nitrogen flow revealed a change in film thickness from 55 to 65 nm.
Case Study 3: In-situ XRD by applying voltage in VO2 thin films
Authors: Laura Rodriguez (1), José Santiso (1), Jessica Padilla-Pantoja (1), Jose Manuel Caicedo (1), Felip Sandiumenge (2), and Gustau Català (1)(3)
(1) Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
(2) Institut de Ciència de Materials de Barcelona, ICMAB-CSIC, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain
(3) ICREA-Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain.
Vanadium dioxide (VO2) has a structural phase transition (SPT) coupled to a metal-insulator-transition (MIT) near room temperature, around 68°C. Below this critical temperature (Tc), VO2 stabilizes in a monoclinic(M1)-insulating phase but if temperature increases up to Tc, VO2 turns into rutile(R)-metallic.
Strain plays an important role in the SPT-MIT behaviour, thus it is important to track both transitions simultaneously when VO2 is in a thin-film form, for instance. Here we are able to do any x-ray diffraction characterization temperature-dependent at the same time that electrical changes are recorded. In figure 13 it is shown the results of one of our experiments where the VO2 reciprocal space maps were taken at different temperatures, while a certain current was injected into the sample and the voltage was measured.

Case Study 4: Chemical Strain Kinetics by Oxygen Surface exchange in epitaxial films explored by Time-Resolved X-ray Diffraction
Authors: Roberto Moreno, Pablo García, James Zapata, Jaume Roqueta, Julienne Chaigneau, and José Santiso
Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
In this study the Nanomaterials Growth Unit developed a new method to study the oxygen surface exchange kinetics in epitaxial thin films (La2NiO4+δ deposited on SrTiO3 (001) substrate) by analyzing cell parameters variations induced by changes in the oxygen stoichiometry of the material. This particular oxide, La2NiO4+δ, is a catalytically active material for oxygen reduction, and a promising candidate to be used as a cathode in SOFC technology
The method consists of continuously analysing the X-ray diffraction pattern of a particular film reflection with a linear X-ray fast detector in static position, while exposing the sample to sudden changes in the pO2 of the atmosphere at elevated temperatures. In this particular study, the evolution of the (-1 -1 10) film reflection along with (-1 -1 3) reflection from SrTiO3 was followed at constant high temperature (600°C) under N2 and air atmospheres, see figure.
This technique demonstrate the use of a conventional lab XRD setup for in situ time-resolved measurements for the chemical expansion (cell parameters changes around 2·10-4 Å) in epitaxial films after variation in the pO2 at elevated temperatures. This capability makes this technique unique to investigate catalytic effects in multilayers and nanocomposite thin films.

Reference: Roberto Moreno et al., Chem. Mater., 25 (18), pp 3640–3647, 2013
DOI: 10.1021/cm401714d
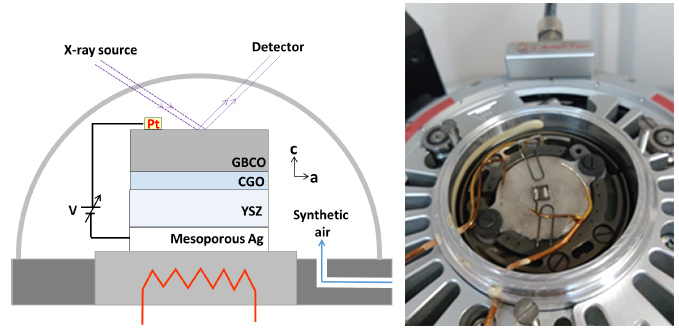
Case Study 5: In-operando study of chemical expansion and oxygen surface exchange in epitaxial GdBaCo2O5.5 films by time-resolved X-ray diffraction
Authors: Arindom Chatterjee, Jose Manuel Caicedo, Belén Ballesteros and José Santiso
Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
The oxidation and reduction mechanisms in solid oxide fuel cells (SOFCs) are gaining much interest in the last years in order to develop an advanced technology in electrochemical devices. The present study focuses on the determination of the mechanisms of chemical strain and oxygen surface exchange in a mixed ionic electronic conducting GdBaCo2O5.5 ±δ (GBCO) material with a layered perovskite structure.
In this study the Nanomaterials Growth Unit developed a new method to study the oxygen surface exchange kinetics in epitaxial thin films by applying an electric field between the electrode material (GBCO) and a solid electrolyte (YSZ), while making use of the time-resolved XRD technique without changes in the pO2 gas atmosphere.
The structure cell parameters of the GBCO material (c-axis parameter) were continuously monitored by XRD, while simultaneously a positive to negative voltage vias (0, ±100, ±200 and ±300 mV) were applied to the electrodes. Results show cell parameters changes of about 10-4 Å during the oxidation and reduction processes in an epitaxial thin film, associated with the oxygen surface exchange rates.
Reference: Arindom Chatterjee et al., J. Mater. Chem. A, 6, 12430-12439, 2018
DOI: 10.1039/C8TA02790K
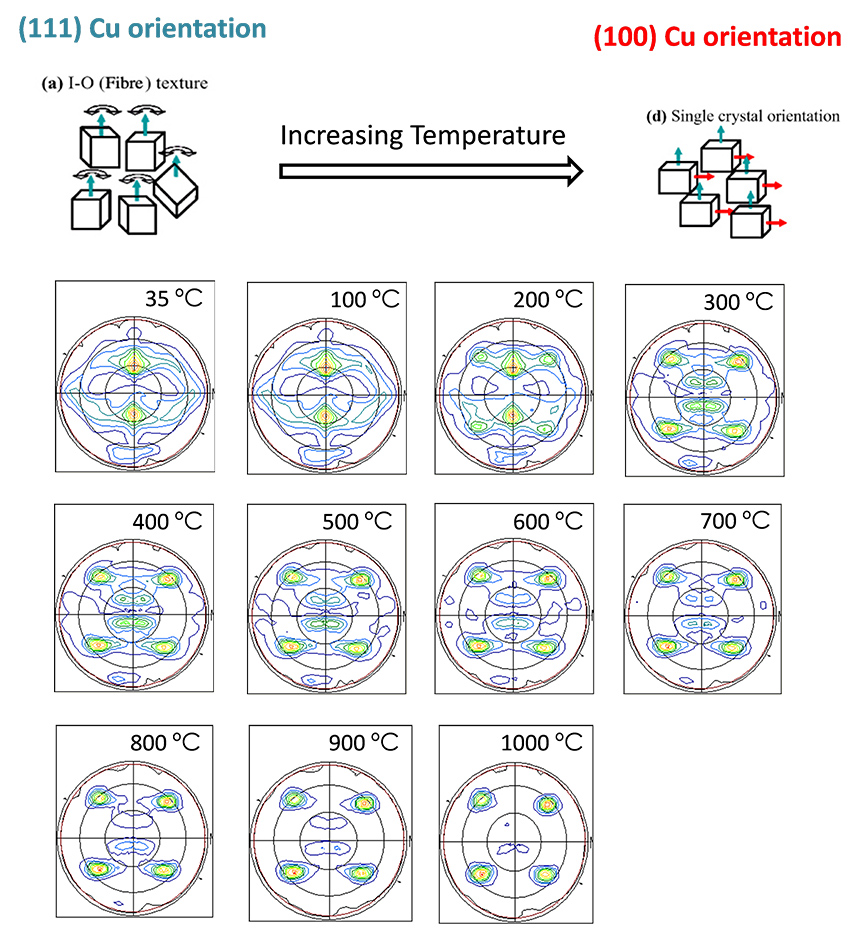
Case Study 6: Textures changes in Cu films under high temperature
Authors: Elena Xuriguera
Universitat de Barcelona, Dept. de Ciència dels Materials i Química Física, Facultat de Química, 08028- Barcelona (Spain)
Cu is an interesting substrate for High Temperature Superconductors (HTS) thin films for its low cost compared to other metallic substrates, and for its low resistivity.
In this particular study, which is a collaboration of Elena Xuriguera from UB, we use different Cu films with a particular orientation at room temperature, in this case (111) Cu orientation. Here, textures measurements (Pole Figures) were monitored while temperature was applied from room temperature to 1000°C, under vacuum atmosphere. The X-ray diffraction characterization evidence how the Cu film changes from (111) to (100) up to 900°C.
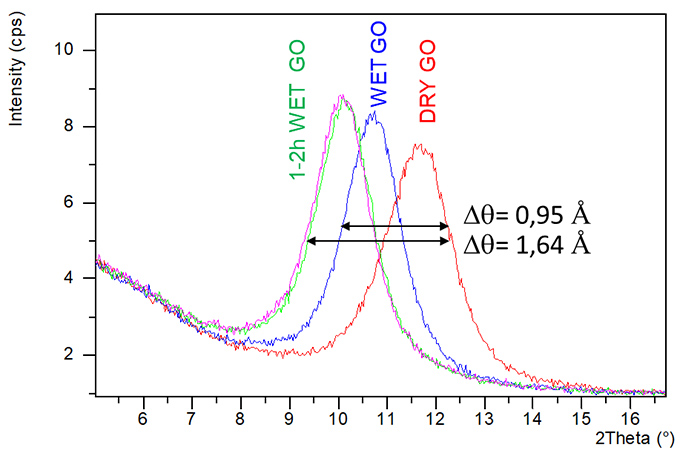
Case Study 7: Hydration behaviour of Graphene Oxide (GO)
Authors: Damià Viana, Steven Walston and José A. Garrido
Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
Neuroprosthetic technology in medicine aims to restore nervous system functionality in cases of severe degeneration or damage by recording or stimulating the electrical activity of the nervous tissue. Currently available neuroprostheses offer a modest clinical efficacy partly due to the limitations posed by the metals used at the electrical interface with the tissue. Such materials compromise interfacing resolution, and therefore functional restoration, with performance and stability.
The Advanced Electronic Materials and Devices group are working to present flexible neural implants based on a biocompatible nanostructured porous graphene thin film that provides a stable and high performance bidirectional neural interface.
The XRD in-situ measurements allows to characterize the graphene thin film under two different atmospheres (dry and wet) to observe the cell evolution in different conditions. The results show how the oxide Graphene peak moves to low angles applying wet atmosphere, indicating an expansion of the cell parameter due to the hydration of the Graphene thin film.
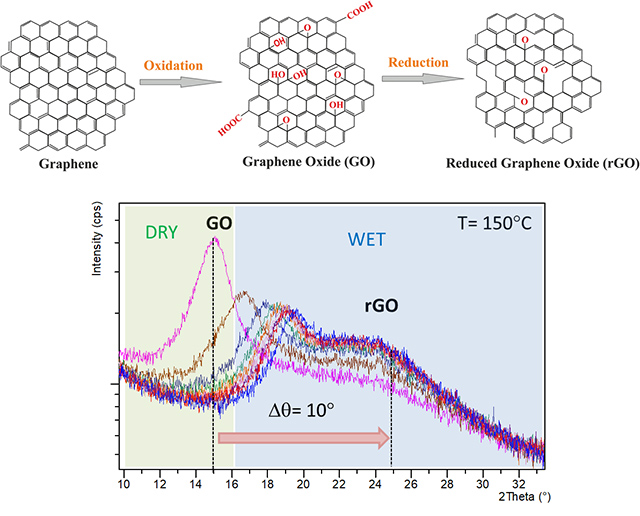
Case Study 8: Monitoring the Reduction Process in Graphene Oxide (GO) by in-situ XRD
Authors: Damià Viana, Steven Walston and José A. Garrido
Institut Català de Nanociencia i Nanotecnologia,ICN2, Campus Univ. de Bellaterra, E-08193, Bellaterra, Spain.
Following the experiment previously described, the nanostructured Graphene film (based on a stack of reduced Graphene oxide flakes) was investigated using in-situ XRD. In this case the stacking distance between flakes was monitored while the GO sample was being thermally reduced at 150°C under wet Nitrogen for hours. The results evidence how the GO peak evolves over time to the reduced GO film peak, showing a decrease from 8.1 Å to 3.9 Å
The Advanced Electronic Materials and Devices group attribute this decrease to the removal of the oxygen groups from the basal plane of the flakes.